Projects
-
Genetic and molecular mechanisms of treatment resistance in DLBCL
Funding: Terry Fox Research Institute
Collaborators: Christian Steidl, David Scott, Nathalie Johnson
Assignees: Chris Rushton, Laura Hilton, Jeffrey Tang, Miguel Alcaide, Aixiang Jiang
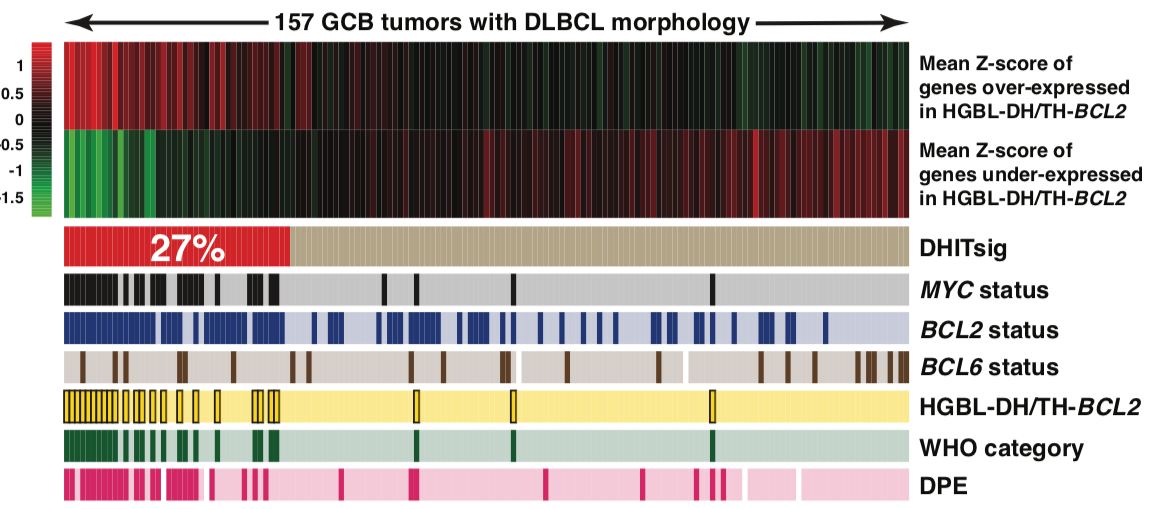
Non-Hodgkin lymphomas (NHL) represent the sixth most commonly diagnosed type of cancer in Canada with diffuse large B-cell lymphoma (DLBCL) being the most prevalent. DLBCL patients exhibit variable responses to available therapies such as R-CHOP. Some biomarkers beginning to enter clinical trials rely on gene expression signatures to identify high-risk cases. For example, DLBCL comprises two cell-of-origin (COO) subgroups with activated B-cell (ABC) cases typically having worse prognosis than germinal centre B-cell (GCB) cases. Therapeutics that exploit molecular features unique to each subgroup are being actively pursued but up-front identification of patients unlikely to achieve a durable response remains a challenge. Although existing methods can identify specific prognostic gene expression features, these capture only some of the clinical heterogeneity and require tumour RNA, which is not always available. Using comprehensive genomic analyses and by integrating mutation and expression data from over 2000 DLBCL tumours, we seek novel molecular features that are associated with treatment resistance in DLBCL. The ultimate goal of this work is to develop new predictive and prognostic biomarkers and identify new therapeutic targets for treatment-resistant DLBCLs.
-
The role of the Fc Gamma receptor locus in resistance to R-CHOP
Funding: ASH Foundation
Collaborators: David Scott
Assignees: Laura Hilton, Miguel Alcaide, Jack Hillman
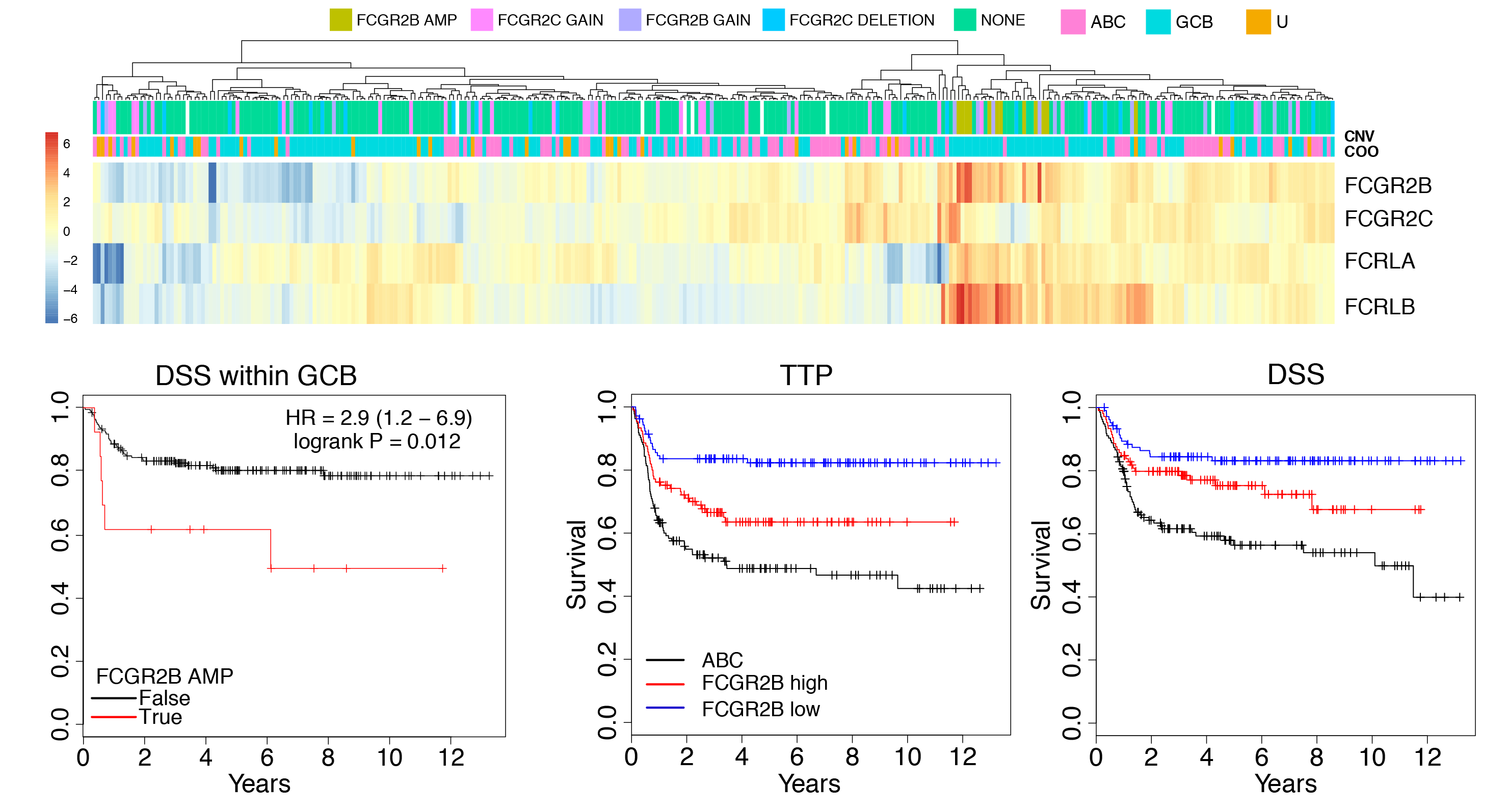
Through genomic characterization of DLBCL we identified somatic amplification of the Fc gamma receptor 2B gene (FCGR2B) as a feature in ~5% of cases. We also found the expression of FCGR2B (by RNA-seq) was prognostic in multiple DLBCL cohorts. FCGR2B contributes to treatment resistance by promoting the internalization of the rituximab antibody by malignant cells, allowing them to evade immune detection. It may therefore serve as an important biomarker to identify high-risk patients and stratify them to recieve newer immunotherapies that can mitigate the effects of high FCGR2B expression. Understanding the genetic mechanisms that drive FCGR2B amplification and overexpression has been complicated by the segmental duplication that affects the FCGR locus, which makes it difficult to accurately align short reads to FCGR2B or its closely-related paralog FCGR2C. We have been employing long-read Oxford Nanopore sequencing and Bionano optical mapping to accurately characterize the structural and single nucleotide variation within the locus.
-
Resolving clonal structure and evolution patterns in DLBCL
Funding: Terry Fox Research Institute
Collaborators: Christian Steidl, David Scott, Nathalie Johnson
Assignees: Chris Rushton, Miguel Alcaide, Sarah Arthur
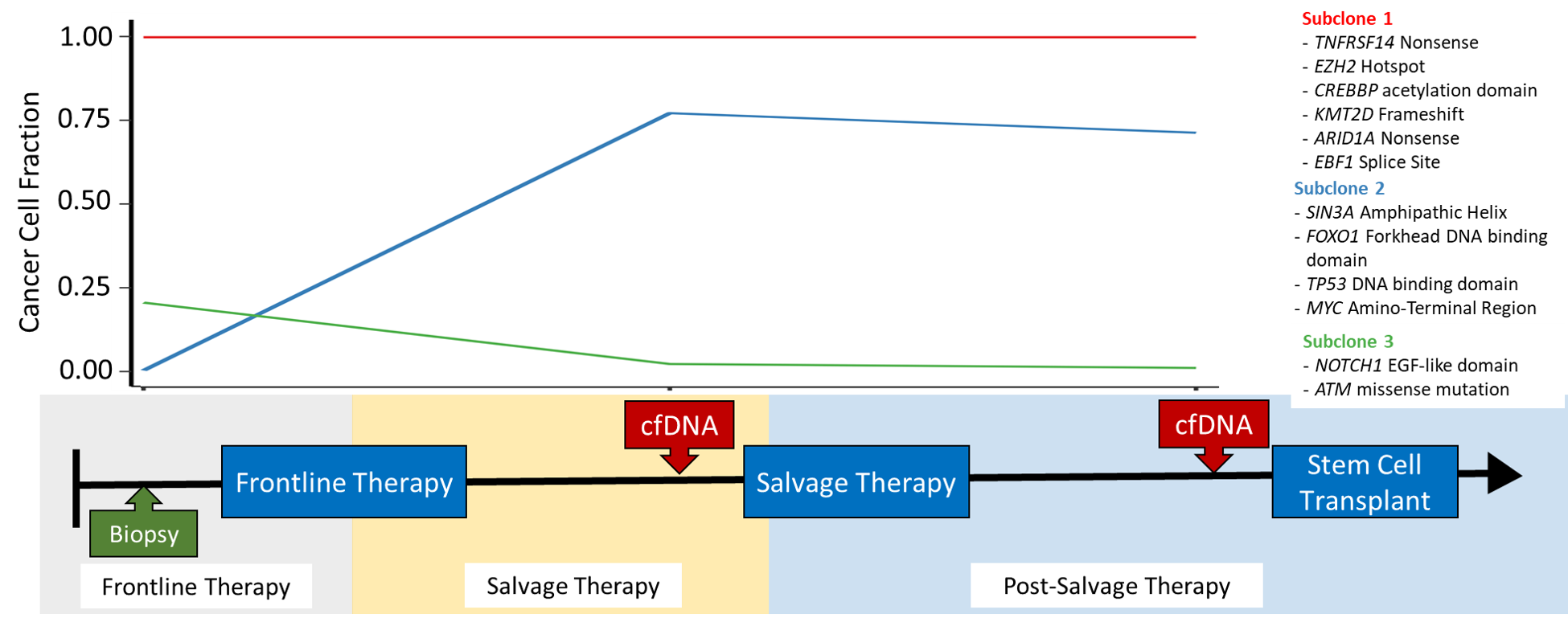
Diffuse Large B-Cell Lymphoma is a genetically heterogeneous form of cancer, with different cells in the tumour acquiring different mutations. This intra-tumour heterogeneity can have profound effects on treatment; if a clonal subpopulations is resistant to treatment, it will reform the tumour following treatment, causing the patient to relapse. We are recieving samples from several clinical trials exploring different salvage therapies for patients with relapsed DLBCL. Tumour biopsies and liquid biopsies are being collected from patients both before and after they are treated. Using these temporal sources of tumour DNA, we can identify clonal subpopulations in the tumour, and further explore how the tumour evolves and adapts in the face of a strong selective pressure such as treatment. The mutations unique to these resistant clonal subpopulations can be explored to determine which mutation(s) result in treatment resistance. We can further determine if these mutations are detectable prior to treatment, which may allow patient to be screened for resistance mutations before they are treated.
-
Circulating Tumour DNA as a biomarker in non-Hodgkin lymphoma
Funding: CIHR
Collaborators: Christian Steidl, Nathalie Johnson
Assignees: Miguel Alcaide, Stephen Yu, Chris Rushton
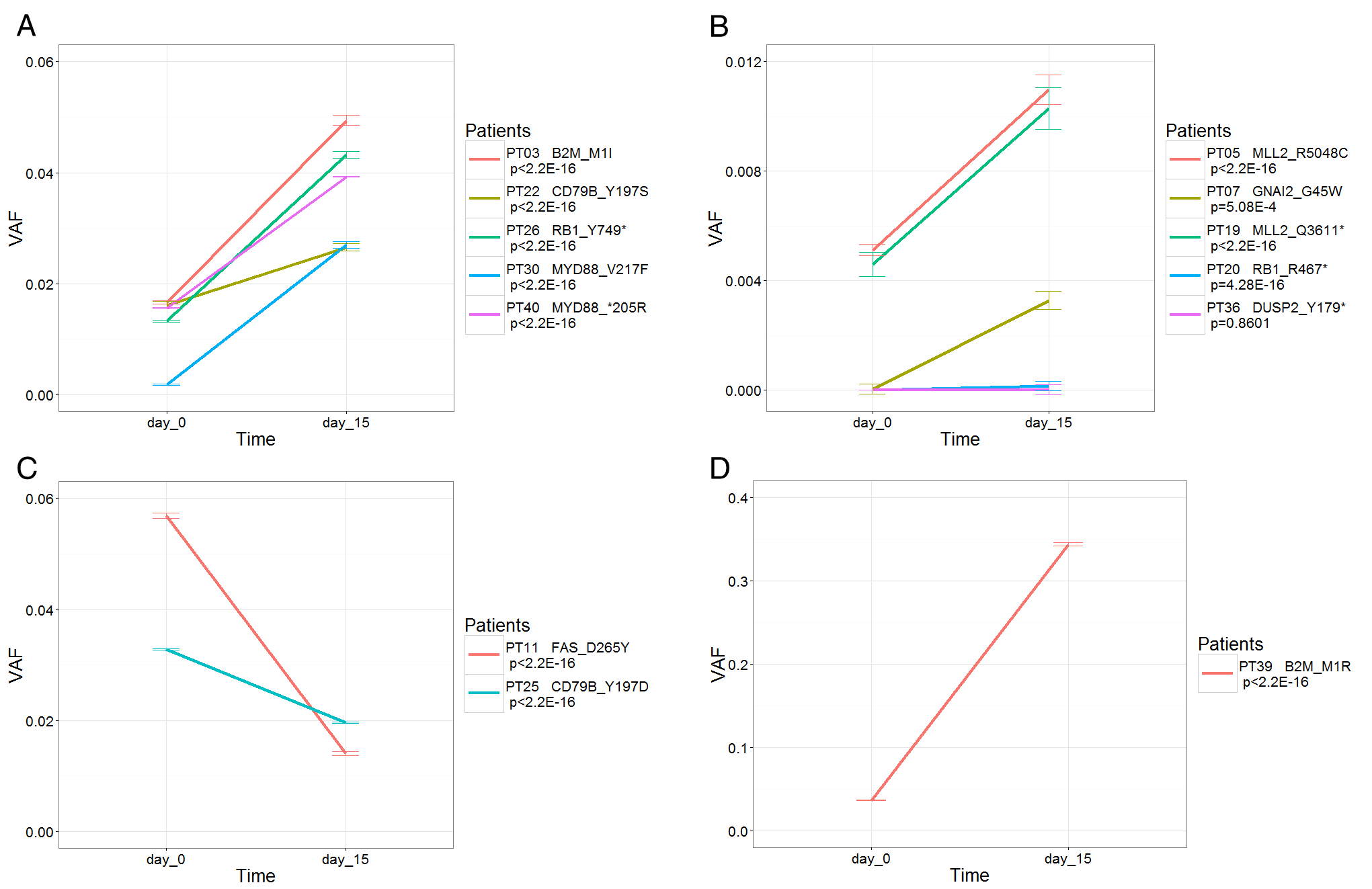
This project aims to develop a novel way of monitoring patients with lymphoma and find a new immunotherapy regimen that would be effective in patients that are not cured with conventional chemotherapy. They will first genetically profile the tumours to determine at which time point they become resistant. They will then determine how these lymphomas evade immune detection and test if targeting these evasion strategies, i.e. "harnessing the immune response" is successful at eradicating chemo-resistant lymphoma.
-
Comprehensive proteogenomic characterization of mantle cell lymphoma (MCL)
Funding: Terry Fox Research Institute
Collaborators: Marco Marra, David Scott, Diego Villa
Assignees: Prasath Pararajalingam, Krysta Coyle
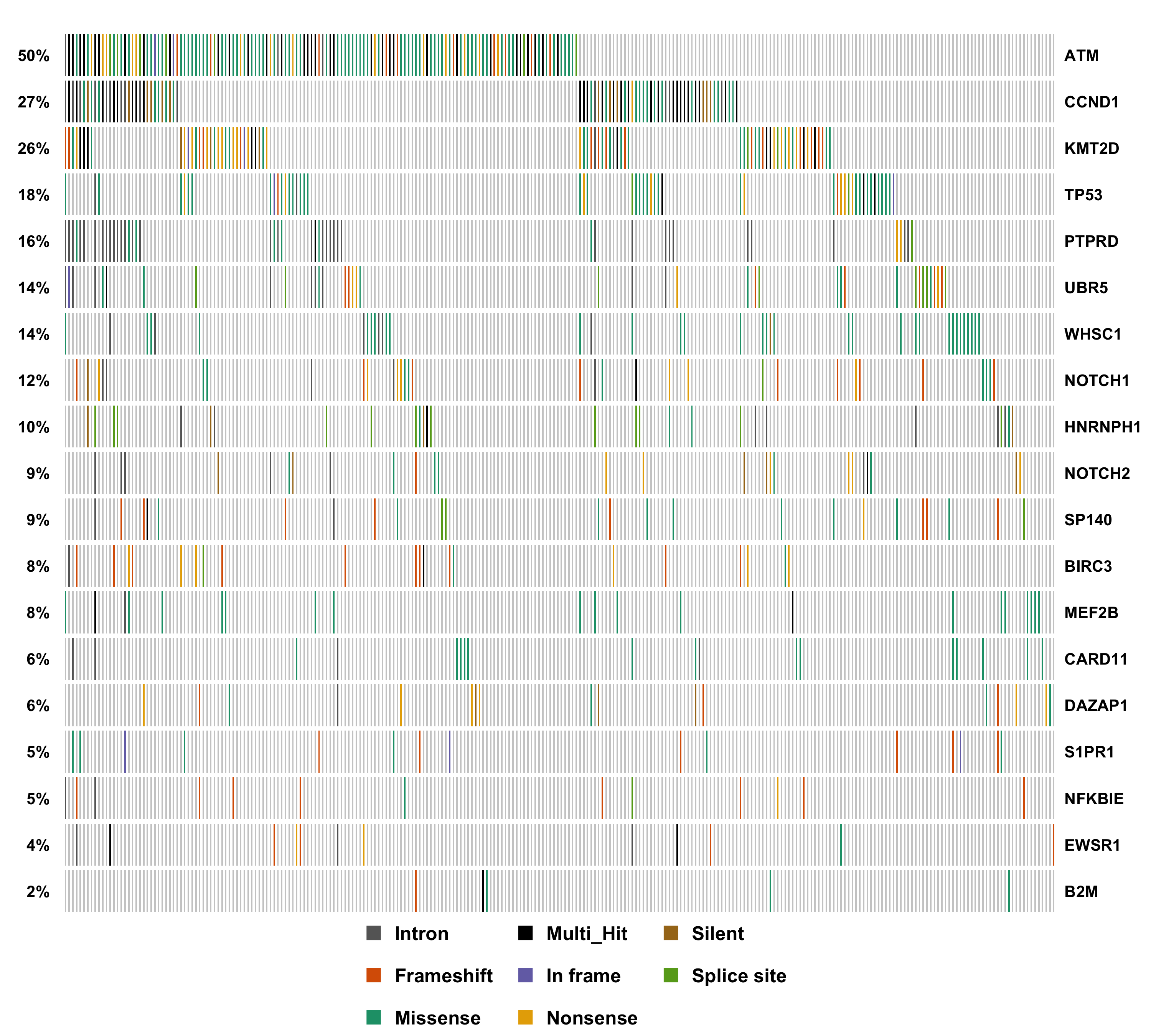
Mantle cell lymphoma is a rare cancer that derives from B lymphocytes in the periphery (mantle zone) of the germinal centre. MCL is most often diagnosed at stage 3 or 4 and is typically aggressive although some patients have an indolent form of the disesae. Only 30-40% of patients with advanced disease achieving a complete response to frontline treatments (R-bendamustine). Heterogeneous clinical response is partially attributable to observable variations in morphology, immunophenotype and proliferative capacity; however, there is little known about the relationship between genetic features and variation in phenotype or response to specific treatment regimens. Through a meta-analysis of in-house and existing exome sequencing data, our group has identified multiple novel driver mutations in MCL including some that associate with high or low proliferation. To more completely ascertain the mutations that lead to lymphomagenesis in MCL, we are performing an integrative proteogenomic characterization of MCL tumours using a combination of whole genome sequencing, RNA-seq and shotgun proteomics. We will are using these data to identify novel coding and non-coding (i.e. regulatory) driver mutations that affect the natural history of MCL including treatment response.
-
Identification and functional characterization of recurrent non-coding mutations in NHLs
Funding: Terry Fox Research Institute, CIHR
Collaborators: Christian Steidl, Tim Audas, Peter Unrau
Assignees: Sarah Arthur, Krysta Coyle, Matthew Nguyen, Aixiang Jiang
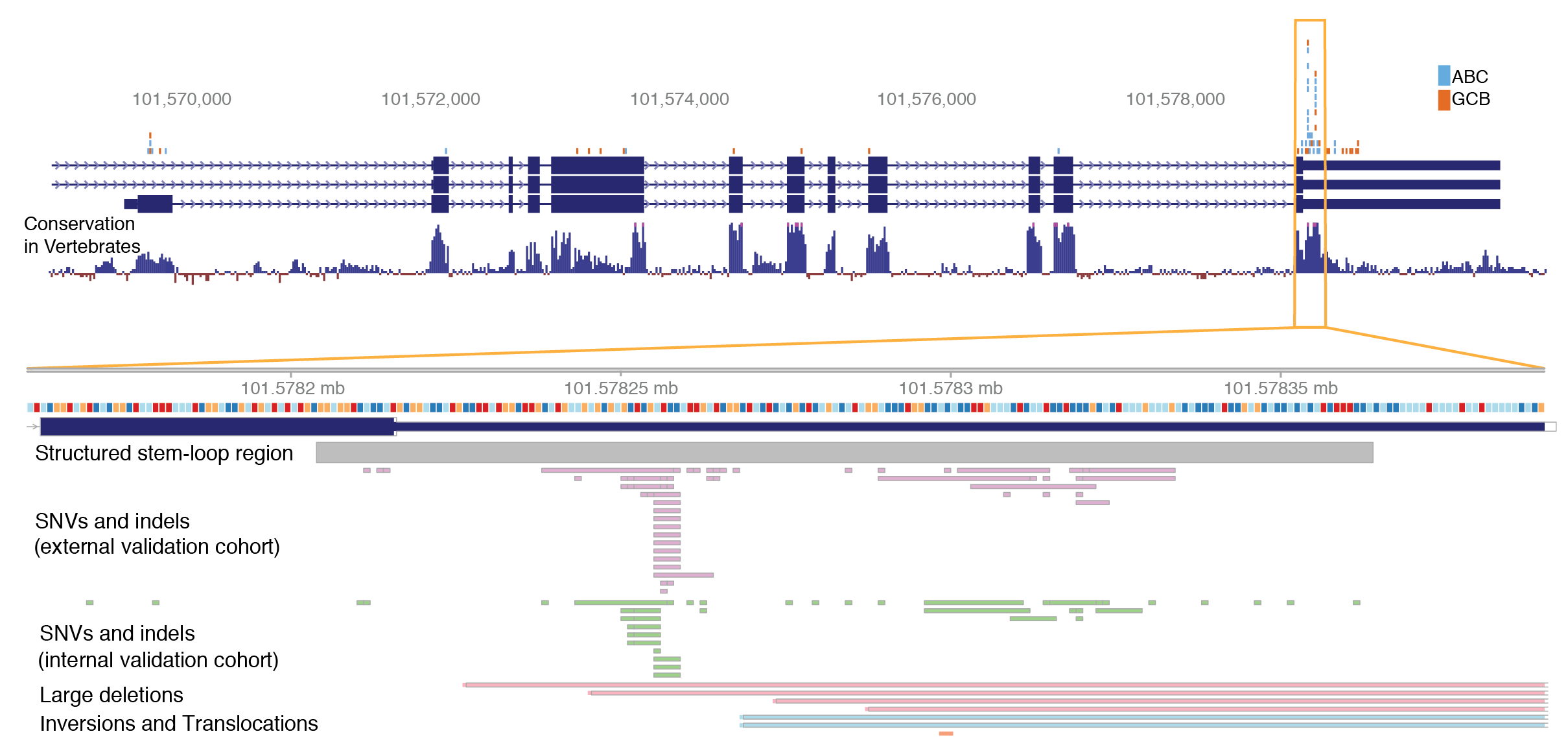
The list of protein-coding drivers identified through sequencing in DLBCL has begun to saturate and yet there remains a gap in our understanding of how these genetic alterations contribute to the transcriptional differences underlying known prognostic signatures and molecular subgroups. For example, dysregulation of NF-κB is the hallmark feature of ABC DLBCL but many ABC cases appear to lack the common driver mutations that are known to promote NF-κB. We are seeking driver mutations that contribute to some features of NHLs. We use data-driven comprehnsive methods that allow identification of non-coding SSMs with a regulatory effect in cis or trans on genes/proteins relevant to malignancy.
-
Comprehensive characterization of endemic and sporadic Burkitt lymphoma
Funding: National Cancer Institute, Foundation for Burkitt Lymphoma Research
Collaborators: Marco Marra, Louis Staudt
Assignees: Bruno Grande, Aixiang Jiang
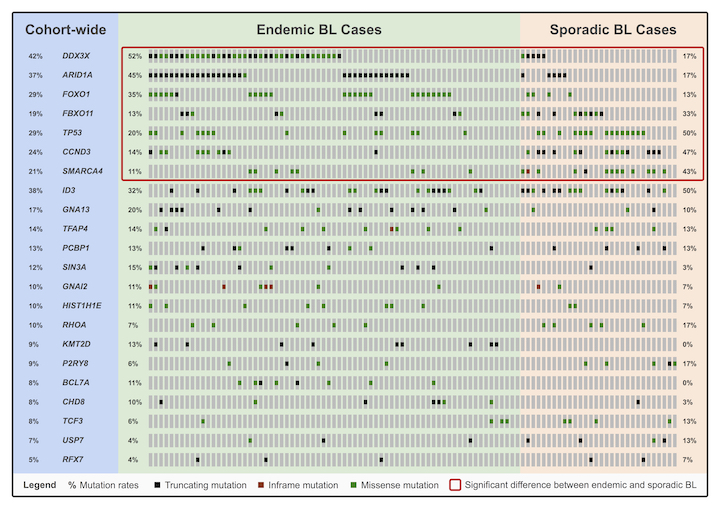
Burkitt lymphoma (BL) is currently classified as one of three clinical variants: endemic BL, sporadic BL, and HIV-associated BL. The endemic variant is primarily diagnosed in sub-Saharan Africa and is epidemiologically associated with the Epstein–Barr virus (EBV) and malaria infection; although, the role of these pathogens in promoting the formation of BL remains unclear. On the other hand, sporadic BL is diagnosed outside of malaria-endemic areas, with only a minority of cases being EBV-positive (10-30%). This project seeks to generate high-throughput whole genome and transcriptome sequencing data from over 100 BL tumours in order to elucidate the genetic and molecular underpinnings of the disease. With this project, we aim to refine the mutational landscape of BL to facilitate the development of less toxic targeted therapies. We also plan on investigating the oncogenic role of EBV and whether it may serve as a better criterion for disease classification than the current system relying on geographic origin.
-
Genetic characterization of HIV-associated DLBCL
Funding: National Cancer Institute
Collaborators: Marco Marra, Louis Staudt
Assignees: Nicole Thomas, Bruno Grande
Diffuse large B-cell lymphoma is a common HIV-associated cancer. The genetic and molecular aetiology of HIV+ DLBCL has not been thoroughly explored. This study seeks to characterize HIV+ DLBCL through comprehensive whole genome, transcriptome, and miRNA sequencing. Enhancing our understanding of the molecular features of these tumours, particularly those that distinguish them from other DLBCLs, may translate into improved therapies for a growing population of patients afflicted with both HIV and cancer.
-
Genetic characterization of canine B-cell lymphomas
Funding: NSERC
Assignees: Kevin Bushell, Jack Hillman, Matt Cheung
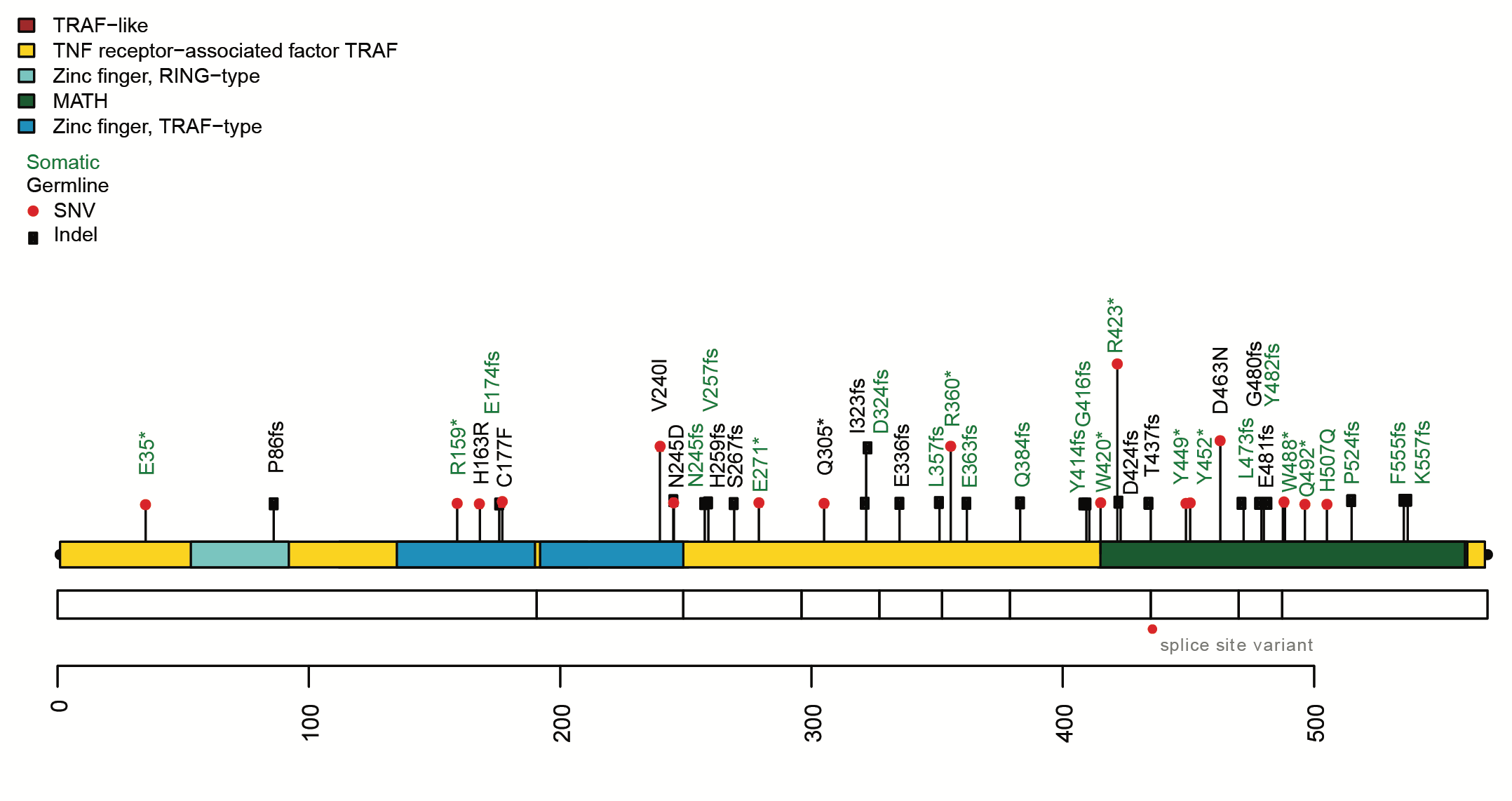
Cancer is the leading cause of premature death in pet dogs and non-Hodgkin lymphomas are the most common cancer in most dog breeds. Unlike human lymphomas with similar histopathology, canine B-cell lymphomas (cBCL) are incurable and exhibit an agressive clinical course. We are sequencing cBCLs to identify driver mutations that contribute to this cancer in a variety of dog breeds. We identified TRAF3 as a novel tumour suppressor in cBCL and are pursuing additional candidate genes. We are also exploring plasma from cBCLs to evaluate the utility of ctDNA as a biomarker and non-invasive source of tumour DNA in canine lymphoma.